Corrosion
Corrosion discribes the reaction of a material with its environment, which leads to a change in its properties and as a result, e.g.a reduction in strength, brittleness or loss of gloss can occur. With metals such as steel, aluminum or zinc, chemical reactions take place on the surface, which cause corresponding corrosion phenomena, such as:
- Surface rust
- Pitting corrosion
- Trough damage
- Crack formation
- White rust of galvanized steel
A special form of corrosion is the electrochemical corrosion of different precious metals in water, which can be influenced by inhibiting the medium. Furthermore, the electrochemical noise during corrosion processes can be investigated with a Potentiostat / Galvanostat.
Besides metals, corrosion can also occurat other materials like glass, ceramics or plastics.
An efficient and inexpensive way to protect substrates from corrosion is the application of coatings. In addition to the barrier effect, these coatings fulfill further functions guaranteed by additives such as active or passive corrosion protection pigments, UV stabilizers or biocides.
In order to achieve maximum effectiveness against corrosion, the right combination and choice of undercoat, pretreatment, functionalization, primer, application method and the appropriate layer structure are crucial during development process. To obtain this perfect combination of protective coating, we carry out customer-specific development orders and consultations.
For investigating the effectiveness of corrosion protection coatings, e.g. paint systems, selected environmental influences such as UV radiation, climate change, temperature, etc. are adjusted in appropriate tests and the samples are checked for changes.
Application Example: Cleaning and Corrosion Inhibition of Cooling Circuits
In some cooling circuits, different metals must be used. When operating with water, this inevitably leads to increased corrosion attacks on the less noble metal and the corresponding deposits cause flow disturbances or failure of the system.
For a cooling system consisting of aluminum alloy, copper and stainless steel, a process was developed in which:
- The circuit is cleaned with inhibited pickling inhibitor to gently remove the corrosion residues
- Further corrosion attacks are reliably prevented by an aqueous inhibiting solution (if necessary with biocide additive)
Method for Detection and Selection
of Suitable Corrosion Inhibitors
by Measuring of Corrosion Current
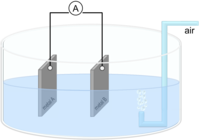
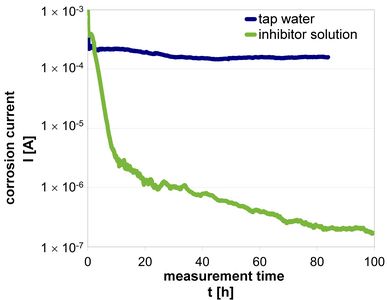
A simple measuring arrangement allows corrosion currents to be determined using a multimeter, thereby providing information on the effectiveness of inhibitors in aqueous solutions. For example, this method was applied for corrosion investigations in the cooling circuit in the presence of various precious metals or to determine the effectiveness of active corrosion protection pigments for use in corrosion protection coatings.
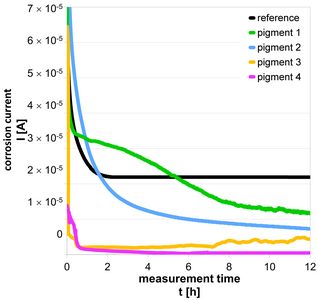
The protective potential of inhibitors, which are added in dissolved form to the surrounding and corrosion-causing medium, can be evaluated by corrosion current measurement. For corrosion-inhibiting pigments, which are to be integrated into a protective coating, potential protective effects in the suspensions can already be differentiated by means of corrosion current measurement.

Dr. Joerg Leuthaeusser
Head of Department
Primer and Chemical Surface Treatment
e-mail
Phone: +49 3641 2825 48