Resorbable gels are used in many areas of regenerative medicine, especially in the field of soft tissue reconstruction. They are also used for wound care, local drug release and as protein or cell carriers. In modern processes, degradable matrices are colonized with the body's own cells and, after implantation in the defect, replaced by the body's own tissue. The favorable swelling properties of polymeric gels are also increasingly used in the cosmetic industry and the field of surgery.
In our department, we produce hydrogels as well as cryogels based on dextran, levan, hyaluronic acid or chondroitin sulfate, but also chitosan- or alginic acid derived gels. By introducing cross-linkable groups into the polymeric starting materials, we are able to vary the chemical and physical properties in such a way that they can be adapted to the respective field of application.
Some of the hydrogels are produced by radical polymerization, which results in gel-like matrices of varying strength. Perforation of the gel allows the formation of continuous channels, which can, for example, ensure the supply of nutrients to cells and facilitate the ingrowth of capillary vessels. By means of 2-photon polymerization, complex three-dimensional structures can be created, which should facilitate the colonization of cells. The biocompatibility and degradability of methacrylic polymer materials and the ingrowth behavior of cells in such hydrogels were investigated in in vivo experiments. Perforation, sandwich construction and pre-colonization with autologous cells showed positive results with regard to the integration of the implants into the surrounding tissue.
Chitosan-based hydrogels can be crosslinked by amide or azomethine formation. Suitable derivatives for these reactions are N-carboxymethyl-chitosan and chitosans with an adjusted degree of deacetylation (DD approx. 50 %), which have an improved solubility behavior under neutral conditions. The cross-linking by amide formation can take place both intramolecularly and by addition of multifunctional carboxylic acid derivatives. For crosslinking by azomethine formation, the chitosan is reacted with polyfunctional aldehydes, e.g. based on polysaccharides (dextran, hyaluronic acid, alginate). The hydrogels are characterized with respect to their degradation/swelling behavior, stability and cytotoxicity.
Another biopolymer that has been neglected up to now is levan, a "polyfructose". This biopolymer is obtained from different bacterial strains (Paenibacillus, Erwinia). Levan can be crosslinked to hydrogels by introducing crosslinkable groups, preferably methacrylate, acrylate, maleate and itaconic acid functions, under light irradiation. Additional functional groups, such as carboxyl, amino or ammonium groups can be introduced to adjust certain properties. Furthermore, esterifications with corresponding acid derivatives of different chain lengths can be carried out using levan. Special derivatives are fluorescence-labeled levans.
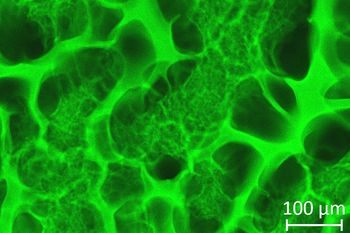
In contrast to hydrogels, cryogels are synthesized from monomeric or polymeric precursors at temperatures below zero degrees Celsius. A three-dimensional network with a system of interconnected macropores in the range of 1 - 100 µm is formed. The advantages of the open pore structure are the efficient transport of nutrients and oxygen and the unhindered removal of metabolic end products.
Our department is specialized in the production of natural biopolymer-based cryogels as matrix materials for the regeneration of soft tissue, as well as for applications in the field of wound healing. In addition to biocompatible, natural polymers based on glycosaminoglycans, synthetic polymers (such as polyethylene glycol derivatives) are also used as stabilizers or additional pore-forming materials. Biopolymers as starting materials for cryogels could therefore be used to produce artificial matrices that correspond to the extracellular matrix not only in structural but also in material terms.
We would be pleased to take over your custom synthesis. Detailed information about the synthesizable compounds can be found here.